More Stories
Hudson Valley counties are modifying vaccine appointments due to the statewide pause on the use of the Johnson & Johnson vaccine.
The Putnam County Department of Health canceled all appointments for that vaccine today at the county’s vaccination site.
The county says all individuals will be offered the Moderna vaccine instead.
Individuals who were registered for a Johnson & Johnson
vaccine today in Garrison can register on site for the Moderna vaccine or
schedule a Moderna appointment online.
For more information click here.
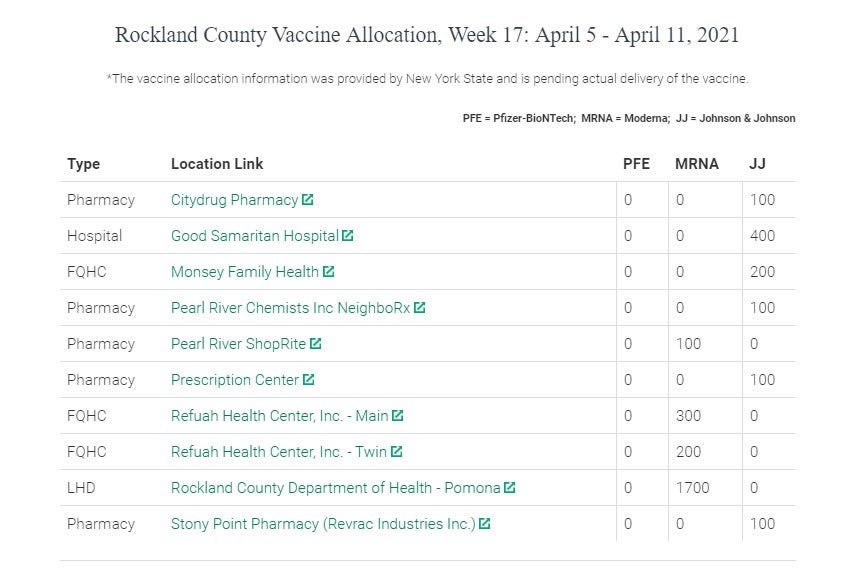
In Rockland County, officials say their Department of Health has not received Johnson & Johnson since Week 14, which was March 15 to 21, so there are not any Johnson & Johnson vaccination appointments currently scheduled at the Pomona clinic.
However, a number of local providers did receive Johnson & Johnson last week. Anyone who was scheduled to receive the Johnson & Johnson vaccine can instead register to receive the Moderna vaccine on Wednesday, April 14 and Thursday, April 15 at the Rockland County Department of Health in Pomona by clicking here.
Westchester County Executive George Latimer announced his county would also pause use of the Johnson & Johnson vaccine. Sites in Westchester will honor Johnson & Johnson appointments using the Pfizer and Moderna vaccines instead.
“The Westchester County Department of Health has suspended use of the Johnson & Johnson COVID-19 vaccine pending further safety information after the CDC and FDA recommended a halt in the use out of an abundance of caution," Latimer said in a statement. “All appointments for Johnson & Johnson vaccines today at New York State mass vaccination sites will be honored by the State with the Pfizer vaccine. The County vaccination locations and field vaccinators will be utilizing the Moderna vaccine until further notice."
SEARCH FOR A CURE: Statistics and State Resources
VACCINE INFORMATION: What you need to know
VACCINE TRACKER: Check the latest vaccine statistics from New York
New York is following the recommendation from the Centers for Disease Control and Prevention and the Food and Drug Administration and pausing the use of the Johnson & Johnson vaccine statewide, due to safety concerns. The CDC and FDA will review data involving six reported U.S. cases of a rare and severe type of blood clot in individuals after receiving the Johnson & Johnson vaccine.
More from News 12
2:04

Viral search underway for 'Sprinkles' after Rock Hill girl loses beloved stuffed bunny on Metro-North
1:34

Stony Point's first female town supervisor begins first official week
1:47

Courtroom packed as man beaten by Peekskill police officer in viral video is arraigned on 4 charges
2:12

Peekskill man sentenced to 23 years to life for killing social worker
0:31

Norwalk woman arrested for stealing bar mitzvah money
